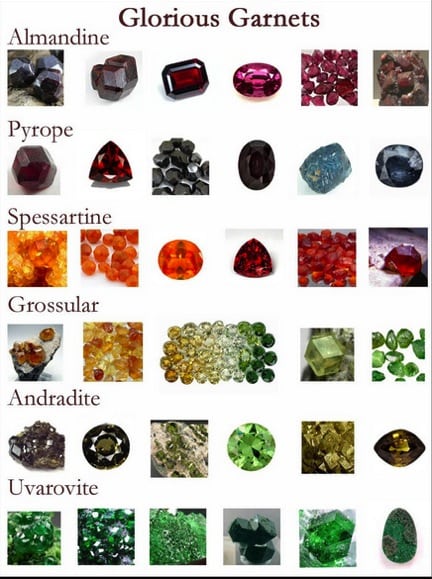
In this blog, the subject is the Garnet Family and their six members, (See Figure 1), which are gemstones and mineral specimens highly prized by collectors, as well as by those that wear them, because of their colors. Their colors, as seen in Figure 1, span the rainbow, and some unexpectedly undergo a change in color, depending on the wavelength of the light, (daylight or incandescent light), that illuminates them, as seen in Figure 2.
I will discuss the relationships between the colors of the garnets and their chemical compositions and how their colors are perceived, due to absorption of light by specific colors and by included chemical species. Such as a tomato absorbing mostly green light and reflecting red light. As an example of another optical effect, (and other gemstones can exhibit this effect also), I’ll discuss asterism, the cause of the formation of a star-shaped figure, seen on the surface of some Star Almandine garnets.

Since antiquity garnets have been admired and worn as gemstones in jewelry such as: the 7th century Anglo Saxon sword pommel found as part of the Staffordshire Hoard in July 2009, [Fig 3]; And in Figure 4, the 1st -2nd century Roman necklace; and in Figure 5 the Roman ear rings; and in Figure 6, the 30 – 323 BCE Egyptian ring; the Byzantine pendant shown in Figure 7; and the Victorian necklace shown in Figure 8. References for each piece of jewelry accompany its picture.






The chemical relationships between the six, end members, of the garnet family are summarized in the two, phase diagrams, shown below in Figure 9, [Ref 11]. The shaded regions of each diagram show compositions where the metal ions, calcium (Ca), magnesium (Mg), and iron (Fe), can substitute for each other. For example, increasing substitution of Mg2+ for Fe2+ in Almandine leads to a garnet increasingly approaching, and ultimately equal to, Pyrope in composition. Appreciation of the shaded regions of mixed composition is important in relating colors of the gemstone to the relevant metals presented in its ionic form, as shown in Tables 1 and 2.
The formulas of the end members of each of the families are also shown in Table 1. The formula cited for the end member Uvarovite, in Figure 9, is erroneous in this old version, (The only one I could dig up), of its compositional group. Only the metals calcium (Ca) and chromium (Cr) are present in this garnet.
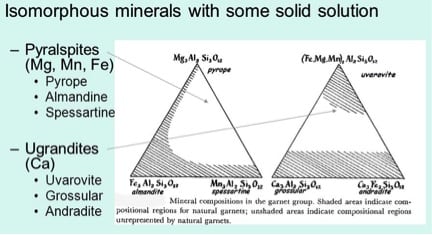
The three garnets of first group, the Pyralspites, contain the metals magnesium (Mg), Manganese (Mn), and iron (Fe), and aluminum (Al). Each garnet of the other group, the Ugrandites, contains calcium. The other metals are aluminum (Al), iron (Fe), and chromium (Cr). In common, all six members of the garnet family contain the element silicon (Si), bound with Oxygen (O), and are known as silicate minerals. These phase diagrams are from an older unreferenced source with an erroneous formula for Uvarovite garnet – Its correct formula can be seen in Table 1.
The formulas for each of the members of the garnet family are listed in Table 1. In the formulas, the charge of the ion of the metal is shown for each metal. For example, the divalent iron ion is written as Fe2+ and the trivalent chromium ion as Cr3+.
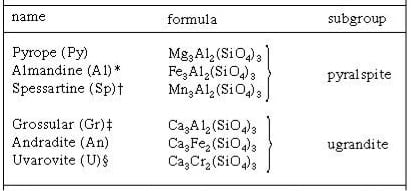
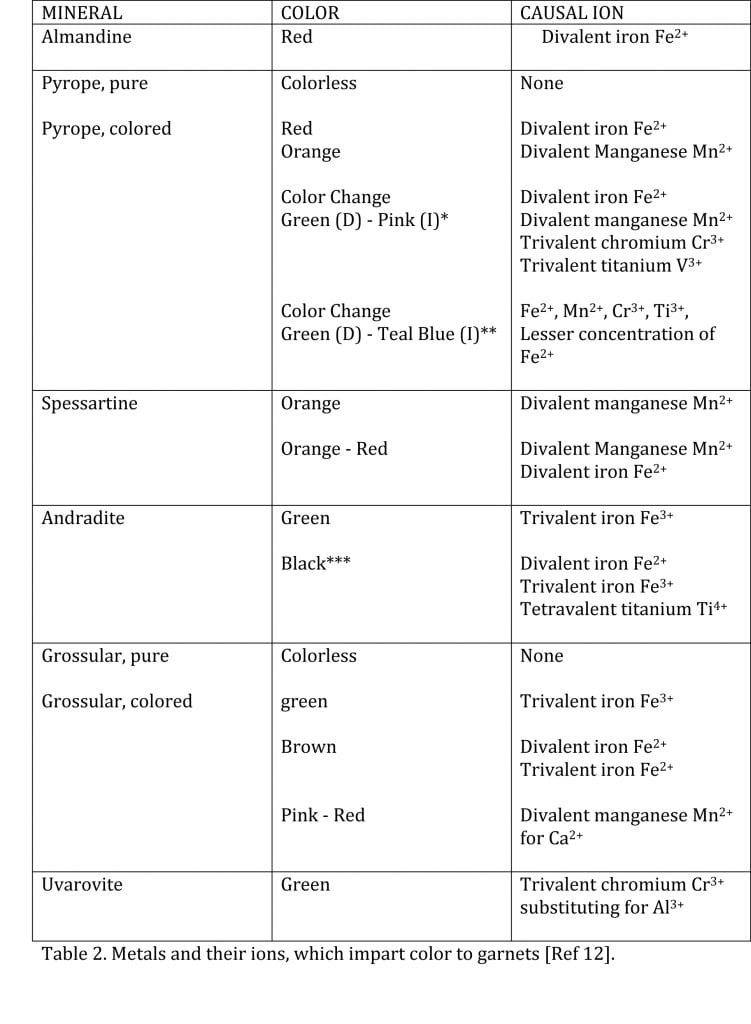
Of the garnet family, Almandine and Andradite owe their colors to the iron present in their formulas, Spessartine to manganese, Uvarovite to chromium, all in ionic form. Pyrope and Grossular, in pure form, are colorless. Various combinations of iron, manganese, titanium, and vanadium ions are the causal agents of color in both Pyrope and Grossular. The formulas for each of the members of the garnet family are summarized in Table 1. The colors; the responsible metal; and its ion forms, which are present in garnets, are given in Table 2.
HOW GEMSTONE COLORS ARE PERCEIVED
The colors of the light we see, either transmitted or reflected by a gemstone, stem from that part of the spectrum of the incident light which is not absorbed within the gemstone. The light, incident on the gemstone, is ambient light. Depending on the source of the incident light, its intensity over the blue to red range of the spectrum can be weighted more in the blue than in its red regions. As examples, light from a halogen lamp, or a white light diode have a spectrum richer in the blue region than an incandescent lamp which has a spectrum richer in the red region.
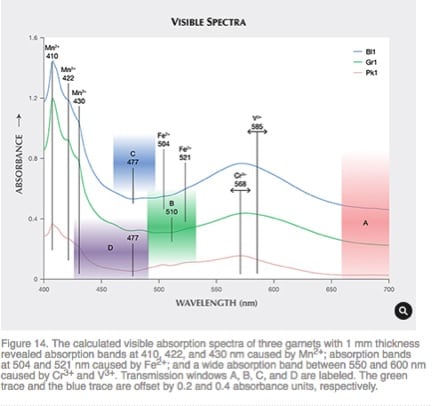
The effects of the spectral content of the incident light and its absorption at some wavelengths and not at others on perceived color can be demonstrated in a study done on color change in a Pyrope garnet [Ref 13]. Changes in the colors of a gemstone with illumination lend drama to the gemstone. A study was undertaken of a type of Pyrope color-change garnet from Tanzania so that the thickness of a cut gem was optimized in a way that the color change, with a change in illumination, could be maximized.

Some gemstones, such as the Almandine garnet, Moonstone, Spinel, Rose Quartz, Citrine, Diopside, Emerald, Sapphire and Ruby may exhibit asterism in displaying a rayed star, best viewed when centered on the dome of a cabochon-cut gem, as in Figure 12. The star effect is caused by the scattering of light from nano-sized crystals of the mineral rutile, oriented in parallel fashion to each other [Ref 14]. In Almandine garnet the star may be either 4 or 6-rayed according to which directions in the crystallographic lattice the rutile crystals are located [Ref 15]. Star garnets are typically a purple shade, as seen in Figures 12 & 13. Idaho and India are the major, if not the only suppliers, of starred garnets [Ref 16].