The beautiful blue hues of aquamarine, the rich yellows of heliodor, the deep green of emerald, the subtle pinks of morganite, the deep blue of the maxixe beryl, and the rich color of the red beryl (bixbite) span the rainbow, as shown in Figures 1 and 2. The beauty of these gemstones underlies their use by artisans in the crafting of jewelry and art objects, and it’s importance to collectors.
In this blog, I’ll describe Beryl’s crystal structure and the structural defects and chemical impurities which are the sources of color and fluorescence in beryl. I will also present a summary of sources for beryl gemstones and a photo gallery of mineral specimens from around the world, which I found to be not only aesthetic, but representative of the crystal habits of beryl and demonstrated the color variations and fluorescence in beryl gems.

COLORS OF BERYL
Beryl is a beryllium aluminum silicate with the formula BE3Al2(Si6O18) [Ref 2].
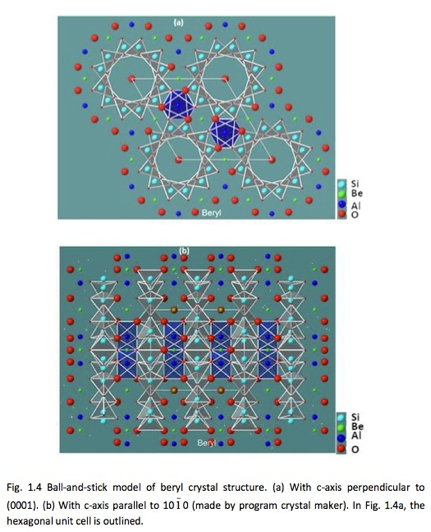
Substitution of other metals as impurities for the intrinsic beryllium and aluminum in the crystal lattice of beryl, are the causes of color in beryl. The positions of beryllium, silicon and aluminum ions in the crystal lattice of beryl are shown in Figure 3 [Ref 3]. The divalent beryllium ion (Be2+) is in coordination with four neighboring oxygen atoms, (tetrahedral coordination), and the aluminum ion (Al3+)in coordination with eight oxygen ions, (octahedral coordination). Twelve silicate tetrahedrons are arranged in circular patterns forming rings. The vertical stacking of the 2-D lattice shown in Figures 3.a results in a stacked ring arrangement which forms channels, as shown in Figure 3.b. Water molecules and alkali metal ions, like the monovalent sodium ion Na1+ are located in the channels [Ref 4].
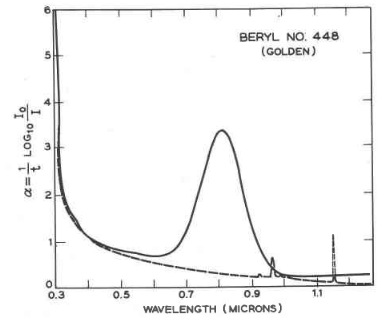
The impurity metal ions and free radical anion responsible for the colors of beryl gemstones are presented in Table I. The process resulting in the absorption of light is electron transfer from a neighboring oxygen to the metal ion. The absorption of light, in regions of the visible light spectrum, defines other wavelengths of the spectrum where the absorption is small and is transmitted or reflected. As an example, the absorption spectrum of heliodor, the golden beryl, taken in polarized light and shown in Figure 4, show a wide absorption peak at ~810 nm in the near infrared part of the spectrum and the ascending flank of a rising peak at a wavelength less than 300 nm in the ultraviolet. Comparison of the wavelength region with low absorption of light, with the colors of the visible spectrum displayed in Figure 5, show the transmitted or reflected light to fall in the yellow-orange range of the spectrum.
TABLE I Impurity Ions and Associated Colors in Beryl[Ref 4,Ref 5,Ref 6]
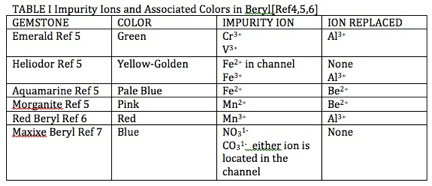
Al3+ = trivalent ion of aluminum,
V3+ = trivalent vanadium ion,
Fe2+ = divalent ion of iron,
Fe3+ = trivalent ion of iron,
Be2+ = divalent ion of beryllium,
Mn2+ = divalent ion of manganese,
Mn3+ = trivalent ion of manganese,
NO31- = nitrate radical ion,
CO31- = carbonate radical ion
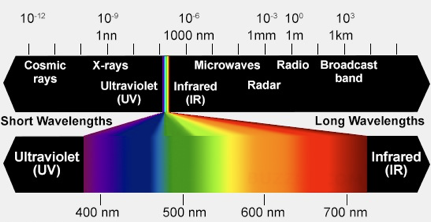
FLUORESCENCE OF BERYL
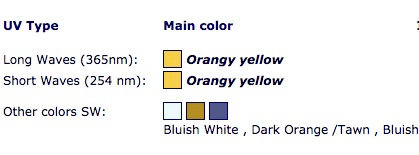
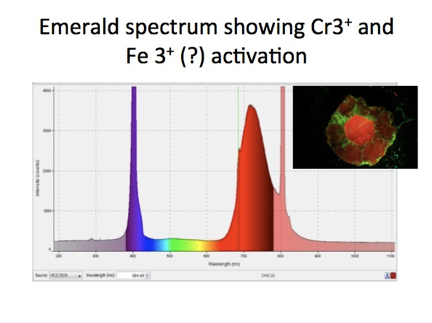
All metal impurity ions listed in Table I fluoresce upon UV light absorption, and serve as activators of fluorescence in beryl. Colors of fluorescence which have been observed in beryl with excitation by a UV lamp are shown in Figure 6 [Ref 6]. Emeralds also exhibit red fluorescence as shown in Figure 4, but require the intensity of laser light for visible fluorescence to be observed. [Ref 7, Ref 8]. The reason for the requirement of intense light for activation stems from the effects of the surrounding lattice about the chromium ion Cr2+ which increases the energy density, (intensity), of light required to excite the electron transitions responsible for fluorescence. A UV lamp is sufficient to excite the chromium ion in ruby because the effect of the lattice on the electrons of the ion is less in ruby.
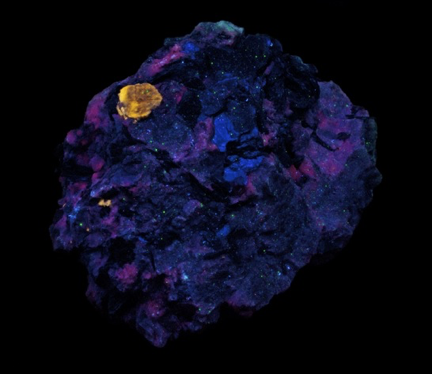
GALLERY OF FLUORESCENT BERYL SPECIMENS

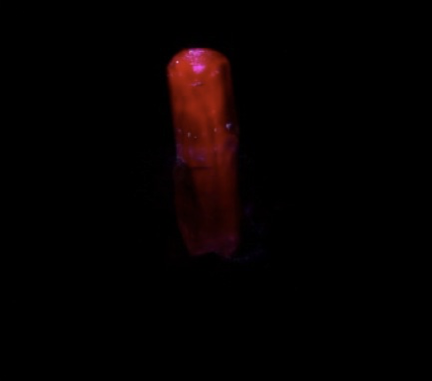

SOURCES OF BERYL GEMS
Sources of gem varieties of beryl extend around the world and are summarized in Tables II and III. Many sources of gem-beryl crystals are a cavity in a
Pegmatite [Ref 5] located within granite [Ref 6]. Others are located in rocks associated with pegmatites. Red beryl is found in the extrusive igneous rock rhyolite. Differently, the emeralds are found in veins of calcite in shale in the mines at Muzo, Colombia. Worldwide locations are summarized in Tables II and III.
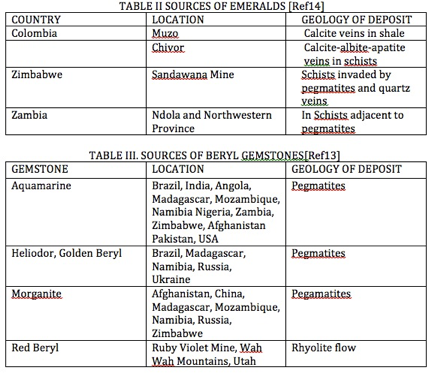
Sources of Beryl Gemstones [Ref 13]
GALLERY OF GEM BERYL
The specimens shown in Figures 6-18 were selected to demonstrate the variations in the forms of gem beryl crystal and the variations in the characteristic colors of gem beryl. The crystal forms range from a hexagonal prism terminated by a flat face as in Figures 6-11, to prisms terminated by one or more families or pyramidal faces as in Figures 12-18, and to crystals exhibiting higher order prismatic faces as in Figure 11.






Figure 13. Morganite on feldspar, Chapa Dara District, Konar Province, Afghanistan [Ref 22]. Six narrow pyramidal faces truncate the flat termination of the tabular crystal. The peach tone of the crystals stems from the presence of the ferric ion Fe3+ which imparts the golden color of heliodor to the pink coloration of morganite as seen in Figure18.



Figure 15. Aquamarine crystal with pyramidal forms modifying the flat termination [Ref 24]. The slight green tone of the crystal stems from the presence of the ferric ion Fe3+.



REFERENCES
Ref 1. https://www.gia.edu/gia-gem-project-beryl
Ref 2. https://www.mindat.org/min-819.html
Ref 3. https://www.researchgate.net/publication/310443635_Dissolution_behaviour_of_beryl
Ref 4. https://link.springer.com/article/10.1007/s00269-008-0228-4
Ref 5. http://www.minsocam.org/ammin/AM53/AM53_777.pdf
Ref 6. https://www.fluomin.org/uk/fiche.php?id=237
Ref 7. Slide 18 in PPT
Investigation of Gem Materials using 405nm Laser Spectroscopy.pptx
Ref 8. https://www.researchgate.net/publication/235471211_Origin_of_the_different_color_of_ruby_and_emerald
Ref 9.
https://www.worthpoint.com/worthopedia/bb-goshenite-ultra-fluorescent-beryl-1468637379
Ref 10. http://tozourfluorescentrocks.net/2d/Page16RugglesMine/BerylmatrixRugglesMineGraftonNH2SW.html
Ref 11. https://www.fluomin.org/uk/fiche.php?id=803
Ref 12. https://geology.com/rocks/pegmatite.shtml
Ref 13. https://geology.com/rocks/granite.shtml
Ref 14. http://www.geo.utexas.edu/courses/347k/redesign/Gem_Notes/Beryl/beryl_triple_frame.htm
Ref 15. https://www.irocks.com/minerals/specimen/8277
Ref 16. https://www.irocks.com/minerals/specimen/41903
Ref 17. http://www.mineralmasterpiece.com/Sold_Specimen_p3.html
Ref 18. https://www.pinterest.com/pin/323203710752245026/
Ref 19. https://collectorsedge.com/pages/the-kagem-emerald-mine-kafubu-area-zambia
Ref 20. http://www.johnbetts-fineminerals.com/jhbnyc/gifs/72325.htm
Ref 21. https://www.mindat.org/photo-144867.html
Ref 22. https://www.mindat.org/photo-231277.html
Ref 23. https://www.mineral-forum.com/message-board/viewtopic.php?p=52056
Ref 24. http://www.johnbetts-fineminerals.com/jhbnyc/mineralmuseum/picshow.php?id=48048
Ref 25. https://www.saphiraminerals.com/Search?page=6&updateId=10
Ref 26. http://www.mineralmasterpiece.com/Sold_Specimen_p14.html