In some minerals color is directly related to a metallic element, is characteristic, and can be useful in identification. As examples, azurite as shown in Figure 1A, is always blue due to the presence of copper, and rhodochrosite, shown in Figure 1B, is always pink to red due to the presence of manganese,. However minerals such as fluorite, colorless in it self, can be yellow, blue, purple, or green due to low concentrations of metal impurities.
Accordingly color alone, generally is insufficient for identification, but when linked with knowledge of other properties of a mineral can be useful in its identification.
Understanding of the roles of metals as sources of colors in a mineral, particularly when augmented with knowledge of its location where found, can be useful in identification. In this Blog I’ll describe how the presence of a transition metal element, present intrinsically or as an impurity, and certain structural features within the mineral are sources of colors or when present, can modify the appearance of the mineral.


Field, Northern Cape, South Africa[Ref2]
CAUSES 0F COLORS AND COLOR EFFECTS IN MINERALS
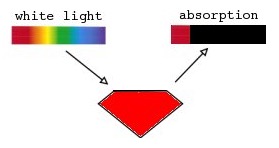
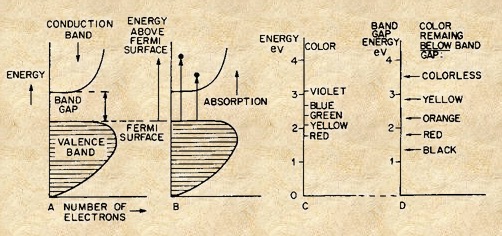
The color of a mineral that we see arises from transmission of light over the visible wavelengths of light within the visible light spectrum which are framed by one or more regions of wavelengths that are absorbed as shown in Figure 1C.
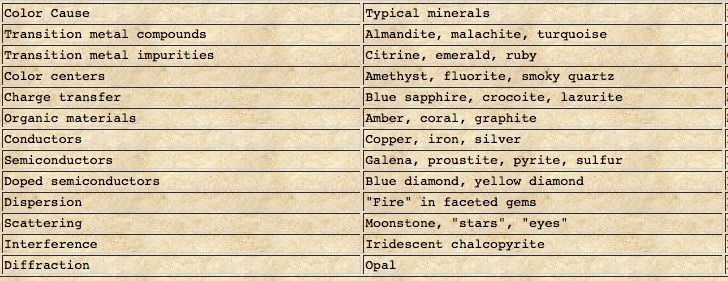
Extensive studies have shown that light absorption by a number of processes are among the causes of the colors in minerals as summarized in TABLE I[Ref4].
Absorption of light by electrons in transition metals present intrinsically or as impurities are involved in light absorption processes and the excitation of electrons within their intrinsic energy distribution within conductors and semiconductors upon light absorption also imparts colors in other minerals which are conductors and semiconductors. Excitation of an electrons or a hole (hole = absence of a negatively charged ion or electron) associated with color centers which reside in defects within the crystal lattice imparts colors in some minerals
Structural features in minerals with sizes of the order of the wavelengths cause physical optical effects: light scattering, interference, and diffraction of light. These can either introduces colors or add optical features to the appearance of the mineral.
Light Absorption by Transition Metals[Ref4,5,6]
Among the causes of color in minerals listed in TABLE I[Ref4] are the ions of transition metals that contribute to the color of many minerals through selective light absorption over the visible light spectrum. They can be present as a major constituent such as manganese (Mn) in rhodochrosite(MnCO3) giving it its pink-red color, present as a low concentration impurity in a mineral such as chromium (Cr)substituting for aluminum (Al) in Ruby giving its red color and the formula {Al,Crminor)2O3, and as ions of iron, chromium , manganese, titanium(Ti) participating in intervalence charge (electron) transfer between two of their ions and with the oxygen ion[Ref5].
Transition metals are present as a formulaic constituent and some as an impurity are shown along with their associated colors in TABLES II and III.
In a transition metal ion with a partially filled d-shell the electrons in the outer region of the d-shell are unpaired. The surrounding oxygen ions of the crystal lattice exert forces on the outer d-shell electrons dermining their occupied and empty energy levels[Ref5]. The forces and energy levels depend on the strength and nature of the bonding as well as the valence of the transition metal ion. These energy levels are quantized so that a absorption of a specific amount energy is required to to increase an electrons energy to another level. Absorption of light of that energy at the corresponding wavelength provides the energy required for excitation of the unpaired electron to a higher level.
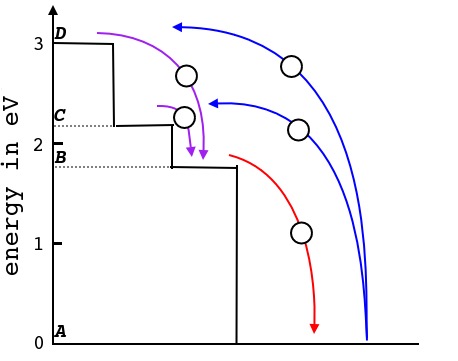
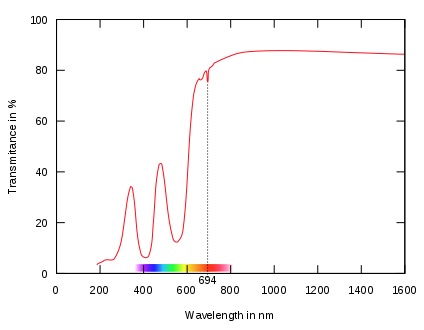
As an example consider absorption and transmission of light in the ruby. The sumation of surrounding forces on the chromium ion Cr3+ result in two energy levels, C and D, avialable for an excited electron as shown in Figure 2[Ref3]. Symmetry conditions deny occupancy of level B by an excited electron [Ref].
As shown in Figure 3 light of 414 and 561 nm wavelengths is absorbed . With abosrbtion of these wavelengths light in the regions of 480 nm and greater than 620 nm are transmitted imparting the red color of the ruby.


Light Absorption By Charge Transfer Between Transition Metal Ions and Between oxygen ions and Transition Metal Ions[Ref7,8].
Transition metals can exist in different valence states. These ions can form covalent bonds with oxygen in which electrons in outer shells can travel between the ions upon being supplied sufficient energy. This can result in charge transfer in the form of an electron upon absorption of light with wavelengths in the Ultraviolet through Visible into Near Infrared light ranges. Charge transfer from an adjacent oxygen to a transition metal ion and charge transfer between neighboring transition metal ions connected by an oxygen atom can occur. The direction of transfer charge is from the ion of least positive valence number to the neighbor of higher valence. For example the deep violet-blue color of cordierite var. Iolite as shown in Figure 4 results from the neighboring absorption peak centered at 600 nm due to charge transfer from a ferrous ion Fe2+ to a neighboring ferric ion Fe3+, and extending through the blue range of the spectrum as shown in Figure 5[Ref10]. Other examples of the processes of charge transfer in minerals with their associated colors are given in TABLE IV.


Note that the wide absorption peak in the 400 to 600 nm range results in a transmission window in the violet.
TABLE IV. CHARGE TRANSFER PROCESSES IN SOME MINERALS WITH THEIR COLORS
| Charge Transfer | Color and Mineral* | Reference |
| Oxygen to Metal Transfer | ||
| O2- Fe3+ | Yellow to Brown: beryl/heliodor, quartz/citrine | 11 |
| O2– Fe4+ | Purple: quartz/amethyst | 11 |
| O2- Cr6+ | Yellow to red: Crocoite | 11 |
| O2- U6+ | Yellow: uranophane | 11 |
| Intervalence Charge Transfer | ||
| Fe2+–O—Fe3+ | Violet: jadeite Blue: lazulite, vivianite, | 11,12 |
| Fe2+–O—Ti4+ | Blue: berlaquamarine, corundum/sapphire, cordierite, amphibole, | 11 |
| Mn2+–O—Ti4+ | Greenish-yellow: tourmaline | 11 |
| Others | ||
| Fe2+Ti4+ and both Fe3+ and Cr3+ | Orange, sapphire | 13 |
*For information on any mineral Google “Mindat.org + Mineral name”
Light Absorption By Color Centers[Ref4]
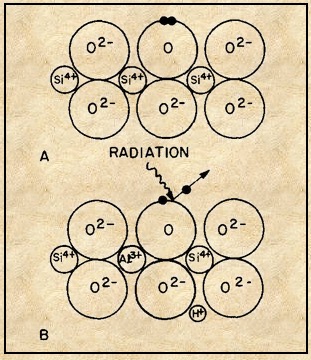
Unpaired electrons can be located on a non-transition metal ion or on a defect in the crystal lattice form color centers as shown in the Fluorite structure and quartz structure shown in Figures 5 and -6. An electron present at a lattice vacancy forms an electron color center and absence of an electron from where an electron pair is normally present forms a hole (lack of a negatively charged ion) center.
In fluorite the absence of a fluorine ion F-1 from its normal position forms an F-center occupied by an electron as shown in Figure 6. Absorption at longer wavelengths by the F-center is responsible for a purple color in fluorite[Ref5].
In smoky quartz the presence of an aluminum ion Al3+ as a low level impurity substituting for a silicon ion Si4+ is required[Ref5]. Removal of an electron from an oxygen ion O2- adjacent to aluminum ions by radiation results in the formation of a singly negative oxygen forming an electron-hole. To preserve charge neutrality a positive hydrogen ion H+ is present. Aluminum not substituting for silicon does not form smoky quartz. The same mechanism accounts for the yellow color of citrine[Ref11].
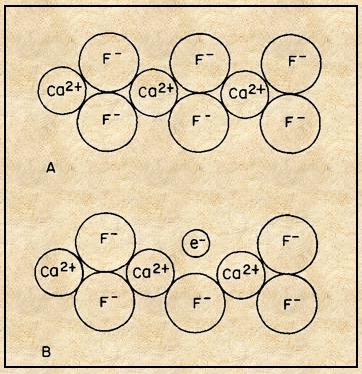
F-center occupied with an electron (B)[Ref5].
Light Absorption in Metals and Semiconductors: Band Theory[Ref5]
The band theory treats electrons as belonging to the crystal as a whole and capable of occupying bands or ranges of energies as shown for metals and semiconductors in Figures 8 and 9.[Ref5]. Colors of representative minerals described by band theory are shown in TABLE IV.
TABLE IV. COLORS OF METALS AND SEMICONDUCTORS DESCRIBED BY BAND THEORY[Ref5].

The Band Theory of Metals[Ref5]
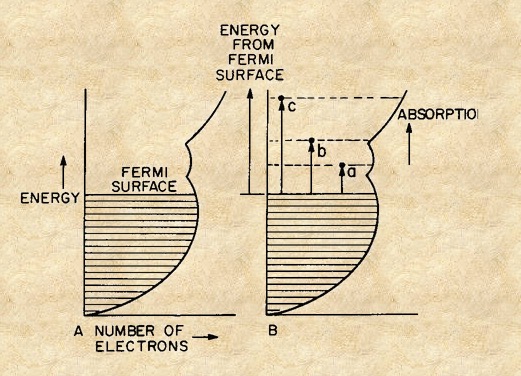
In a metal such as copper or iron each metal atom contributes its outer electrons to a common pool in which they are free to move freely through the crystal accounting for the large electrical conductivity of metals as well as the metallic luster and specular reflection of metals. In a typical metal which contains 1023 electrons per cm-3 all essentially equivalent to each other, such that quantum mechanically
the energy levels are broadened into bands as shown in Figure 8A. The density of electrons each energy level is limited and states are filled to the maximum Fermi surface (level). Upon absorption of light electrons are excited to empty bands of higher energy with density of states varying with as shown in Figure 8B.
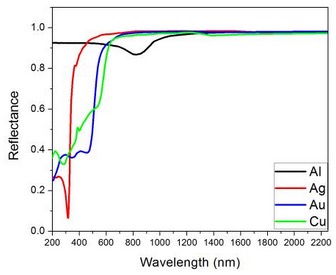
If the efficiency of absorption decreases with increasing energy in the blue-green spectrum as in gold and copper the spectrum features the yellow and reddish colors of gold and copper upon reemission of the electrons as shown by the reflectance spectra shown in Figure 9[Ref18]. The spectra of silver and aluminum in the figure show that light across the visible spectrum is absorbed and re-emitted leading to their white color.



The Band Theory of Semiconductors[Ref5]
Many sulfide minerals are semiconductors that exhibit substantial electrical conductivity, high refractive indices, and often a metallic luster[Ref4]. In minerals where bonding is predominantly covalent or ionic the sulfur electrons occupy a valence band as shown in Figures 13A and 13B. A band comprised of empty metal electron orbitals exists at higher energies separated from the valence band by an energy gap as shown in Figure 13. Absorption of light by electrons of energy greater that the gap energy excites them into the conduction band as shown in the Figures 13C and 13-D. If the band gap is smaller that the energy of the visible light range all of the light is absorbed and the mineral behaves like a metal and appears black or grey as shown in Figures 13–C and13 -D and by tetrahedrite with a band gap of 0.000 eV[Ref21] and galena with a band gap of 0.993 eV[Ref 22] and shown in Figures 14 and 15. As the band gap energy increases the wavelengths excited in the spectrum become shorter and colors shift through red to yellow. The band gaps of those semiconductors such as diamond and sphalerite with band gaps of about 5.5 and 3.5 eV are greater than available energy at the lowest wavelength (highest energy) of the visible spectrum and the light is absorbed. With an intermediate band gaps of 1.99 eV and 2.197eV, respectively cinnabar appears red and orpiment yellow-orange as shown in Figures 16 and 17[Ref23].




Humboldt County, Nevada[Ref22].
Light Absorption and Colors in Impurity and Defect Semiconductors[Ref5]
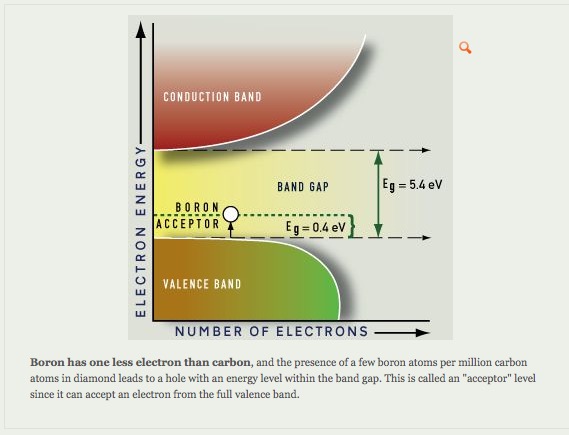
Certain impurities can cause light absorption in large band gap minerals. Absorption of light nitrogen and boron impurity centers as shown in Figure A and –B results, respectively in yellow and blue colored diamonds.
Yellow Diamonds
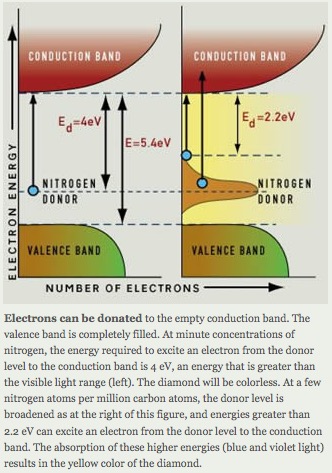
nitrogen impurity results in the yellow color of a diamond[Ref23].

Blue Diamond

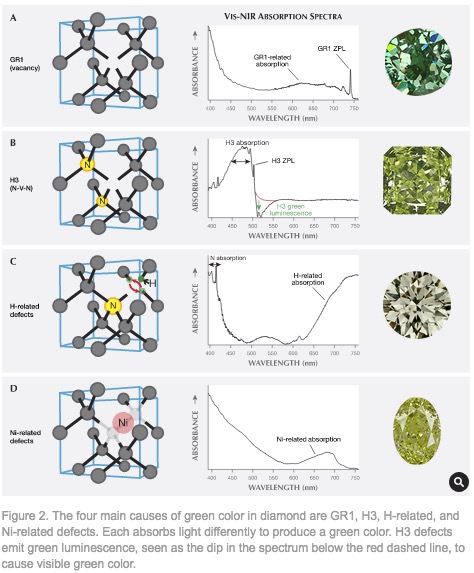
Green Diamond
A green color in a diamond arises from absorption of light in the yellow to red wavelength region by electrons associated with defects in the periodicity of the diamond crystal lattice as shown in Figure [Ref24].
Pink Diamond
Color defect centers developed along deformation planes in the diamond crystal lattice diamond are responsible for the coloration in pink diamonds as shown in Figure23[Ref25,26].

Colors and Features Caused by Physical Optics
Play of color due to structural characteristics of the gemstone can be useful in mineral identification. Iridescence, opalescence and labradorescence, chatoyancy, and asterism are characteristics of a limited number of minerals.
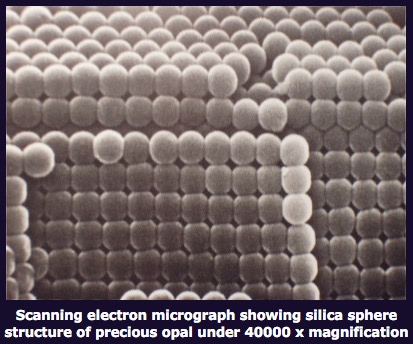
Opalescence
Opalescence is an opal-like play of light producing flashes of different colors as in an opal as shown in Figure 24[Ref40]. The various colors are produced by diffraction from regions with layers of different thickness of formed by silica spheres of different uniform diameters[Ref41]

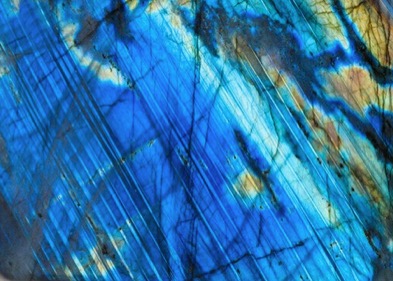
Labradorescence
Labradorite is a feldspar mineral comprised having striations of exsolved lamellae of nanometer of albite in the 10s to 100sof nanometers size range in anorthite with both having different calcium and sodium concentrations. Accordingly their refractive indices are unequal[Ref39,40] and the striations diffract light giving the colors according to the thicknesses of the lamellae giving the labradorescence as shown in Figure 25[Ref44].
Iridescence
Iridescence is the play of colors produced in a thin film of different refractive index than in the surroundings and varying thickness such as the wall of a soap bubble or layer of oil on water[Ref45]. Like diffraction the colors depend on thickness and angle at which viewed. The iridescence of some specimens of the copper mineral chalcopyrite as shown in Figure is a classic example of iridescence on a mineral surface where the colors range from blue to red with increasing thicknesses of the film.

England[Ref34].
Chatoyancy
Chatoyancy is an effect of preferential light scattering by parallel aligned rod like mineral inclusions like the fibers of crocidolite (asbestos) cemented in quartz of the gemstone tigers eye as shown in Figure 28[Ref35,36]. Aligned needle-like microcrystals crystals of rutile are responsible for the chatoyancy of chrysoberyl as shown in Figure .[Ref37]

REFERENCES
Ref 1. https://www.mindat.org/photo-257370.html
Ref 2. https://www.mindat.org/photo-83179.html
Ref 3. http://gemologyproject.com/wiki/index.php?title=Causes_of_color
Ref 4. . https://en.wikipedia.org/wiki/File:Ruby_transmittance.svg
Ref 5. http://www.minsocam.org/msa/collectors_corner/arc/color.htm
Ref 6 https://en.wikipedia.org/wiki/Transition_metal
Ref 8. https://www.gia.edu/doc/SP88A1.pdf
Ref 9. https://www.mindat.org/photo-650677.html
Ref 10. http://minerals.gps.caltech.edu/files/Visible/cordierite/Cordierite2016_India.gif
Ref 11. https://www.gia.edu/doc/SP88A1.pdf
Ref 12. http://minerals.gps.caltech.edu/color_causes/ivct/index.htm
Ref 15. https://www.mindat.org/photo-109627.html
Ref 16. https://www.mindat.org/photo-112379.html
Ref 17. https://www.mindat.org/photo-78563.html
Ref 19. https://materialsproject.org/materials/mp-647164/
Ref 20. https://materialsproject.org/materials/mp-21276/
Ref 21. https://i.pinimg.com/originals/72/26/67/7226674458e6dd34ba15b72f4034130f.jpg
Ref 22. https://en.wikipedia.org/wiki/Hopper_crystal
Ref 23. https://materialsproject.org/materials/mp-641/
Ref 24. https://www.mindat.org/photo-85601.html
Ref 25. https://www.mindat.org/photo-83818.html
Ref 26 https://www.mindat.org/photo-56538.html
Ref 27. http://www.webexhibits.org/causesofcolor/11A0.html
Ref 28. https://www.gia.edu/gems-gemology/spring-2018-natural-color-green-diamonds-beautiful-conundrum
Ref 29. https://www.gia.edu/doc/Characterization-and-Grading-of-Natural-Color-Pink-Diamonds.pdf
Ref 30. https://br.pinterest.com/pin/27092035231593578/
Ref 31. https://www.smithsonianmag.com/travel/the-hope-diamond-102556385/
Ref 32. https://www.pinterest.com/pin/AaGzMFSFFrs9vFLxcvJNl4g6FGLK1W2_i5FGc6i82BuZSmK7fjG7pJ0
Ref 33. http://www.uniqueopals.ch/opal-play-of-colour.htm
Ref 34. http://www.uniqueopals.ch/opal-play-of-colour.htm
Ref 35. https://www.geologyin.com/2016/07/worlds-most-expensive-opal-literally.html
Ref 36. https://www.mindat.org/min-2308.html
Ref 37. https://www.britannica.com/science/exsolution
Ref 38. https://www.google.com/search?client=firefox-b-1-d&q=refractive+index+of+albite
Ref 39. https://www.google.com/search?client=firefox-b-1-d&q=refractive+index+of+anorthite
Ref 40. https://www.sciencedirect.com/topics/medicine-and-dentistry/diffraction-grating
Ref 41. https://austinbeadgallery.com/what-to-look-for-in-labradorite-cabochons/
Ref 42.
https://www.asu.edu/courses/phs208/patternsbb/PiN/rdg/interfere/interfere.shtml
Ref 43. https://www.crystalclassics.co.uk/product/chalcopyrite-(irridescent)/
Ref 44. https://en.wikipedia.org/wiki/Chatoyancy
Ref 45. https://www.asbestos.com/blog/2020/04/20/asbestos-jewelry-mesothelioma/
Ref 46. https://blogs.scientificamerican.com/rosetta-stones/tiger-s-eye-a-deceptive-delight/