Amber is a hard resin formed from tree sap by fossilization and is many millions of years old[Ref1]. Since Neolithic times (about 9000-3000 BC) and before the Copper Age[Ref]2) amber has been highly valued as a gemstone and used to create beautiful jewelry and artworks. Wide use of amber in Early Europe and in the area of the Mediterranean Sea is shown in jewelry and artworks displayed in Figures 2-7. Works from the Medieval Era to the present are shown in Figures 8-13.
In this Blog chemical and physical properties of amber which underlie its beauty and use as a gem are described in descriptions of its chemical and mechanical properties, and the sources of its colors. Also preservation of fossils of insects and other organisms in amber is described briefly.
AMBER IN ANCIENT EUROPE
As shown in Figure 1 amber from sites (red) where amber was found in Ancient Europe were distributed widely from their greatest concentrations along the coast of the Baltic Sea and along the North Sea coast along Jutland. As indicated by the routes indicted in black and red movement of amber could proceed from the North and Baltic Seas to Mediterranean countries terminating in what are now Italy and Greece[Ref]. Collateral routes led to the Black Sea, Syria, and Egypt[Ref3]. Amber was moved further Eastward along the Great Silk Road to China and Southeast Asia[Ref4]. Collateral routes between sites of origin over what is now Europe to the major North-South routes served to distribute locally mined amber around the continent.
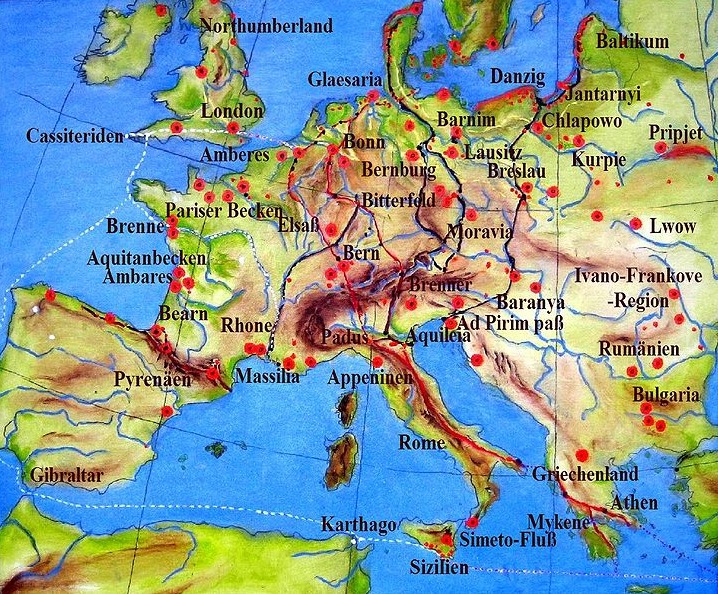
Ancient Europe[Ref3].
AMBER JEWELRY AND ART WORKS FROM ANCIENT TIMES INTO MODERN TIMES












1890-1910[Ref11].

THE CHEMISTRY OF AMBER
The fossilization of tree sap into amber proceeds by crosslinking of di-terpenoid and tri-terpenoid molecules by free-radical polymerization[Ref13,14]. Over the millions of years during which crosslinking occurs to form polymers; polymerization, and cyclisation also occur resulting in new chemical compounds[Ref13,15,16]. The resulting mixture of substances can be described in terms of its carbon, hydrogen, and oxygen contents by the formula C10H16O. Sulfur comprising up to 1% of chemical species may also be present[Ref].
SOURCES OF THE COLORS OF AMBER

AMBER PIGMENTS
Studies[Ref18-27] on specimens of amber gathered from worldwide sources have shown that members of the chemical terpenoid family[Ref13] as well as other chemical species can contribute to the yellow to brown, red, and black colors of amber as shown by specimens of Baltic Amber in Figure 14.
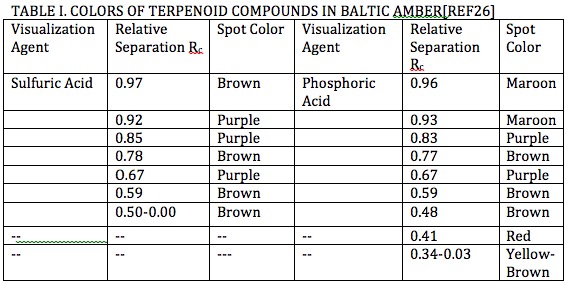
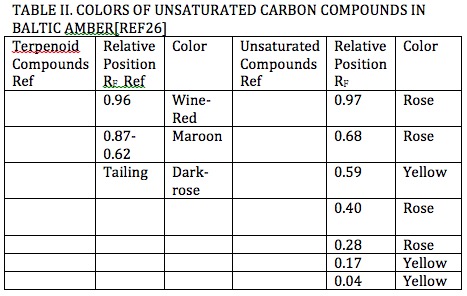
As an example a study conducted on Baltic amber separation by liquid chromatography* showed separation of terpenoids and unsaturated organic compounds showed colors ranging between yellow, shades of red, and brown as shown in Tables I and II [Ref25]. These colors have been found in amber gathered from around the world.
*In separation of chemical species by liquid chromatography[Ref27] specific chemicals are used to sequester other chemical species which transport at different rates during separation by gravity resulting in degrees of separation from their collective position which range between 1.00 and 0.00 as shown by the example in Figure 15 .
AMBER WITH COLOR DUE TO FLUORESCENCE
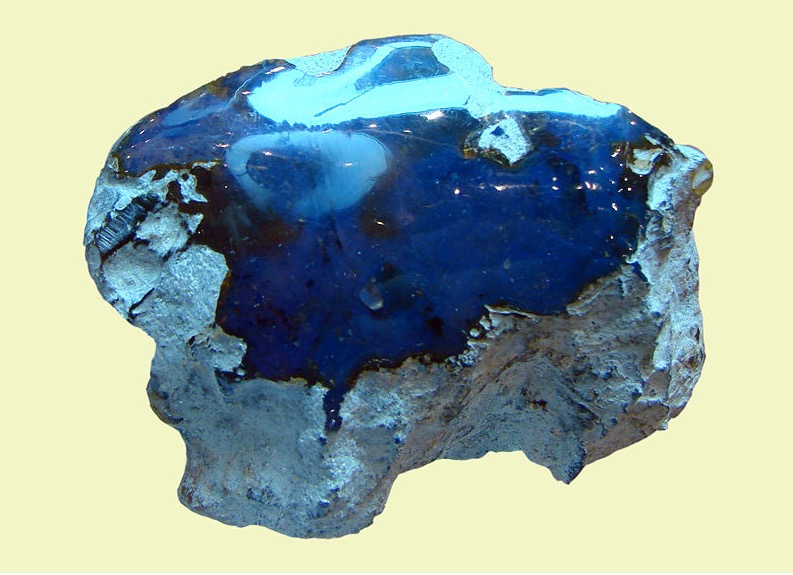
Some amber from the Dominican Republic exhibits a strong Cobalt Blue fluorescenceas shown in Figure 16[Ref28]; some amber found in the Baltic, the Ukraine, Far-Eastern Russia, and Sumatra also exhibit blue fluorescence[[29,32]. Studies on these ambers have shown that the blue fluorescence in ambers from the Dominican Republic and Sumatra stems from the ring-like organic molecule perylene[Ref28,30,31] and that from the amber from Far-Eastern Russia stems from the pyrene eximer[Ref29,34,35].
PHYSICAL PROPERIES OF AMBER
Being an organic resin amber is amorphous with no crystalline structure; accordingly it is not classified as a mineral but as a mineraloid, a mineral-like material[Ref36]. It’s Mohs Hardness is in the range 1-3 and it fractures in brittle fashion but is tough in its tenacity at temperatures near room value.[Ref36]. At
higher temperatures near210°C amber can be bent, allowing repair of amber pipe stems, as well as formed by pressing and sintering pieces together[Ref37.38]. The latter process allows construction of art objects such as a box as shown in Figure 17. [Ref39].

PALEONTOLOGY OF AMBER
Organisms and organic matter such as a feather can be trapped and ultimately over long time become inclusions in amber. With initial entrapment on the surface of amber and subsequent deposit of more amber organism can be entrapped as shown in the sequence in Figures 18 and 19. A great variety of organisms can be preserved as shown in Figure . Following initial and subsequent deposits of sticky amber and entrapment episodes a volume of amber containing fossils is attained[Ref40].

Figure 19 [Ref40].

REFERENCES: AMBER
Ref 1. https://en.wikipedia.org/wiki/Amber
Ref 2. https://www.ancient.eu/Amber/
Ref 3. https://commons.wikimedia.org/wiki/File:Amber_sources_in_Europe.jpg
Ref 4. https://en.wikipedia.org/wiki/Amber_Road
Ref 5. http://museumcatalogues.getty.edu/amber/objects/37/
Ref 6. https://www.pinterest.com/pin/373869206562406397/
Ref 7. https://www.pinterest.com/pin/438397344957150618/
Ref 8. http://shewhoworshipscarlin.tumblr.com/post/134242133432/greyhound-pendant-1600
Ref 9. https://www.1stdibs.com/jewelry/earrings/dangle-earrings/georgian-amber-gold-earrings/id-j_202058/
Ref 10. http://theebonswan.blogspot.com/2014/01/amber-gold-necklace-set-1860-70.html
Ref 12. https://boylerpf.com/products/russian-sterling-silver-gold-vermeil-vintage-amber-earrings
Ref 13. https://www.scienceinschool.org/2011/issue19/amber
Ref 14. https://en.wikipedia.org/wiki/Terpenoid
Ref 15. https://en.wikipedia.org/wiki/Polymerization
Ref 16. https://en.wikipedia.org/wiki/Radical_cyclization
Ref 17. https://en.wikipedia.org/wiki/Amber
Ref 18. https://www.zmescience.com/science/long-process-amber-creation/
Ref 19. https://www.nature.com/articles/s41598-017-09385-w
Ref 20. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0111303
Ref 21. https://www.tandfonline.com/doi/abs/10.1080/08120099.2014.960897
Ref 23. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-50532011000800015
Ref 24. https://www.sciencedirect.com/science/article/pii/S0146638086800250
Ref 25. https://en.wikipedia.org/wiki/Terpenoid
Ref 26. [PDF] Some possibilities of thin layer chromatographic analysis of the molecular phase of Baltic amber and other natural resins
Ref 28. https://commons.wikimedia.org/wiki/File:Ambre_bleu_dominicain_21207.jpg
Ref 29. Blue-fluorescing amber from Cenozoic lignite … – TerraTreasures
Ref 30. https://www.flickr.com/photos/bob_81667/13427898544
Ref 31. https://www.researchgate.net/publication/326740492_Photoluminescence_of_Baltic_amber
Ref 33. https://en.wikipedia.org/wiki/Perylene
Ref 34. https://en.wikipedia.org/wiki/Pyrene
Ref 35. https://en.wikipedia.org/wiki/Excimer
Ref 36. https://en.wikipedia.org/wiki/Mineraloid
Ref 37. https://rebornpipes.com/tag/bending-amber-stems/
Ref 38. https://patents.google.com/patent/US445285
Ref 40. https://www.palaeontologyonline.com/articles/2015/fossil-focus-amber/