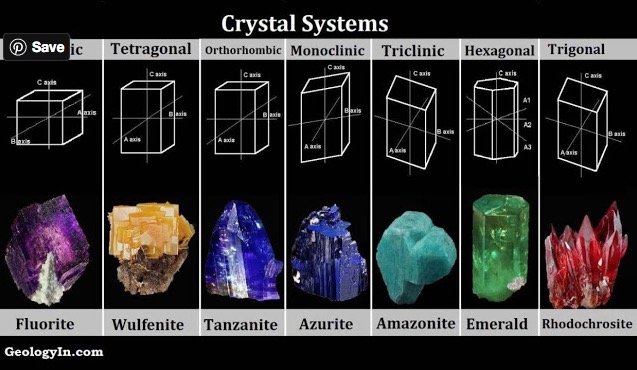
CLEAVAGE IN A MINERAL CRYSTAL[Ref1]
Cleavage in a mineral is the tendency for the crystal to split along definite crystallographic planes as exemplified by the rhombohedron cleaved from a calcite crystal shown in Figure 1[Ref1]. These planes of weakness are present within a regular repeating array of atoms and ions within the crystal and are always parallel to a potential face of the crystal. The weakness arises from the chemical bonds between cleavage planes being fewer in number or weaker than those between ions and atoms within the cleavage planes. Accordingly the crystal will tend to split between the cleavage planes and along a plane of relative weakness. When present, it is the uniqueness of the mode of cleavage in a mineral that makes it a tool in its identification.
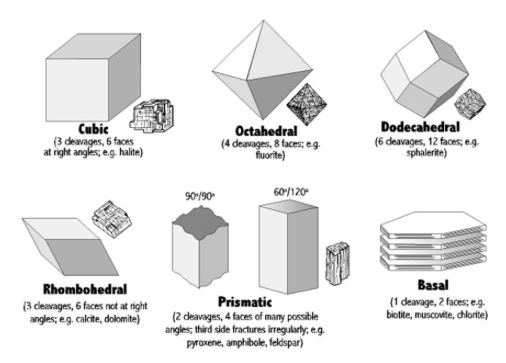
Mineral crystals exhibit modes of cleavage along six families of planes as shown in Figure 1. Identification of the mode of cleavage and descriptions of its appearance and ease of cleaving can be used as steps in the use of Mineral Identification Keys and data compendiums as listed in References 2-7
The excellent YouTube video of Reference 2 shows how to experimentally develop and evaluate modes of cleavage, parting, and fracture in crystals in their identification. Note that use of eye protective wear is a must while performing these experiments.

As demonstrations of the effects on cleavage of the density and strength of chemical bonds crossing the cleavage plane compared to the cleavage plane itself, consider its behavior in the mica family of minerals, in calcite, and in the diamond.
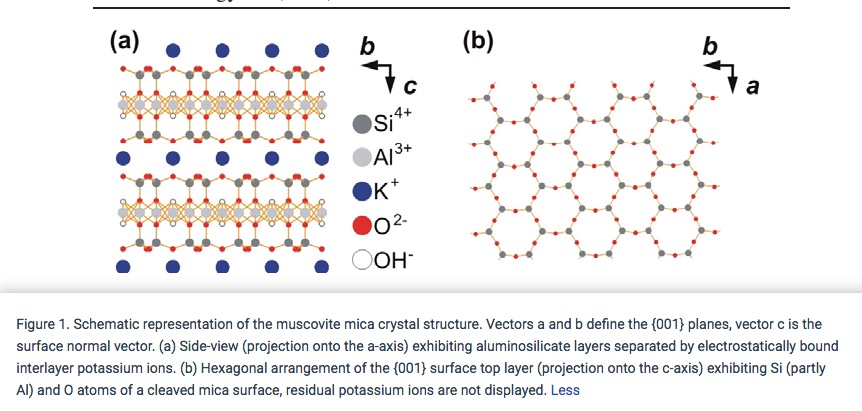
Cleavage in Muscovite Mica
Muscovite of the mica family, as the other members, only cleaves along planes parallel to the basal plane[Ref11] of a crystal with resulting thin sheets as shown in Figure 2. The structural relationship of the cleavage plane to the relative strengths of chemical bonds between the atoms in the lattice of the muscovite crystal is shown in Figure 3.

Cleavage in Calcite
Calcite crystallizes in the trigonal crystal system[Ref15] and cleaves along six planes into rhombohedrons as shown in Figure 4.

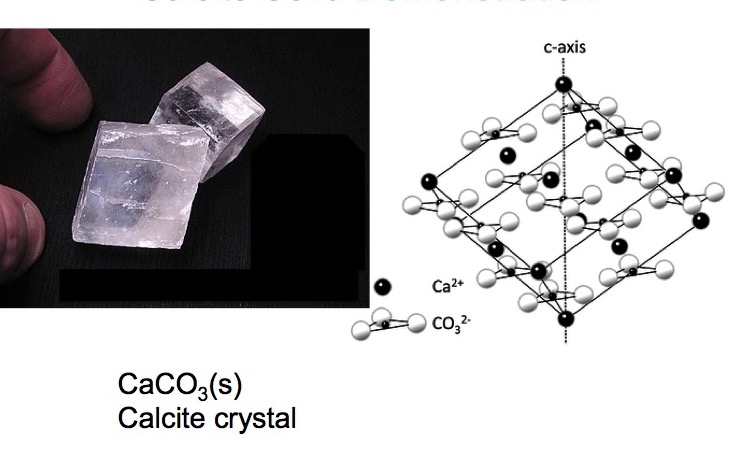
The angles between the legs of the rhombohedral faces are 78.5° and 101.5°.[Ref16] Note that the six cleavage planes lie between extended chain-like arrays of calcium ions and of carbonate ions, and minimal density of bonds crossing the cleavage planes.
Cleavage in Diamond
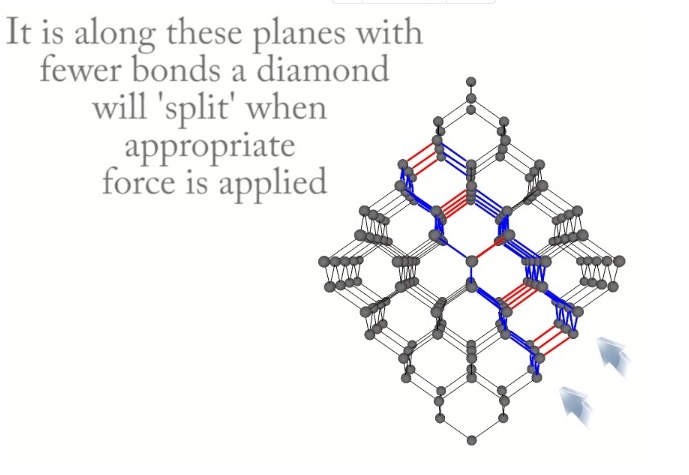
Cleavage in a diamond crystal can proceed any on of eight octahedral planes which parallel the octahedral faces of a diamond crystal as shown in Figures 6-9. The video shown in Reference 17 demonstrates cleaving of a diamond crystal and the relationship the cleavage plane to the density of bonds crossing the plane.
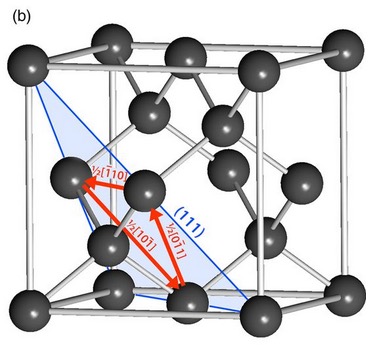
the diagonal octahedral plane is shown in blue[Ref18].
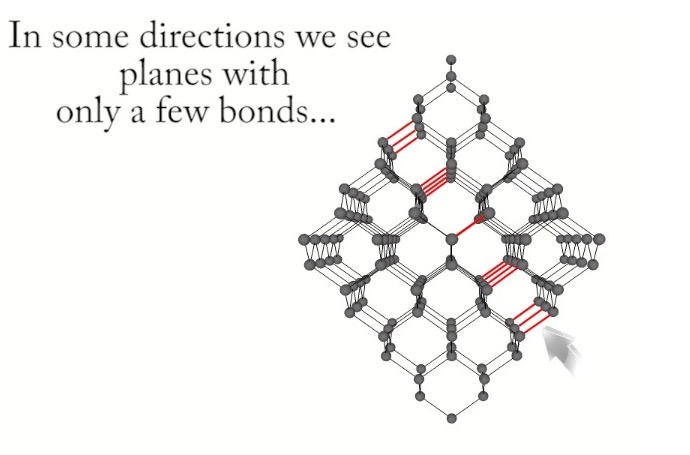
bonds crossing it lying between two parallel octahedral planes, each
with larger densities of atoms and bonds than the plane between them[Ref19].
The parallelism of both a cleavage plane and octahedral faces of a diamond crystal is shown in Figure 9. A representative perfect cleaved surface of a diamond crystal is shown in Figure 10. The white linear features are presne on the outer surface of the cleaved crystal.

Its octahedral faces[Ref20].

Diamond[Ref20].
Descriptors of Cleavage for Use With Mineral Identification
In Mineral Identification Flow Charts [Ref3] and Mineral Data Tables[Ref4,5,6] not only the mode of cleavage, but descriptions of the quality and ease of cleavage for each mineral also are used as part of the body of information used in identification.
Descriptors of the quality of cleavage are summarized in the table in Figure 11. In addition the difficulty in achieving is described as easy, hard, and difficult to produce[Ref3].

REFERENCES
Ref 1. https://en.wikipedia.org/wiki/Cleavage_(crystal)
Ref 2. .http://www.minsocam.org/msa/collectors_corner/id/mineral_id_keyi6.htm
Ref 3. http://www.minsocam.org/msa/collectors_corner/Id/mineral_id_keyi1.htm#TOC
Ref 4. www.mindat.org
Ref 5. http://webmineral.com/
Ref 6. https://www.minerals.net/MineralMain.aspx
Ref 7. Mineralogical Society of America – Mineral-Related Links
Ref 8. https://www.jstor.org/stable/30063973?seq=15#metadata_info_tab_contents
Ref 10. https://images.slideplayer.com/20/5954014/slides/slide_21.jpg
Ref 11. https://www.mindat.org/min-2815.html
Ref 12. https://www.pitt.edu/~cejones/GeoImages/1Minerals/1IgneousMineralz/Micas.html
Ref 14. https://www.mindat.org/min-859.html
Ref 16. https://chemdemos.uoregon.edu/demos/Properties-of-An-Ionic-Salt-0
Ref 17. https://www.britannica.com/science/calcite
Ref 18. https://jgs.lyellcollection.org/content/176/2/337
Ref 19 . https://vimeo.com/20281170