Despite its wide distribution in limestone and as a common gangue mineral in ore deposits, the rainbow of colors and beautiful crystal forms of calcite, from locations around the world, have made it a favorite of collectors. One such favorite is the group of calcite crystals, tipped by hematite, from the Egremont Mine shown in Figure 1. In massive form it has provided lapidists and other artisans a beautiful material for the creation of jewelry and other artworks, such as the sculpture done in Utah calcite, shown in Figure 2.
In this blog I will briefly describe the mechanical properties of calcite, its crystallography, optical properties, and sources of its colors. In Calcite II, I will follow with a gallery of calcite specimens sought by collectors from world-wide locations.

Cumberaland, England [Ref 1]

Mechanical Properties [Ref 3]
The hardness of calcite on the Mohs Scale is 3. It’s brittle because of its perfect cleavage along rhombohedral planes. It parts readily, along twin planes formed by stresses. It can also exhibit conchoidal fracture.
Crystallography [Ref 3, 4]
Calcite crystallizes in the Trigonal System with crystallographic axes, and the often-seen rhombohedral, (Left), and scalenohedral, (Right), forms, shown in Figure 1 [Ref 4]. The system possesses three a1,2,3-axes at 120 degrees with respect to each other in the horizontal plane and the perpendicular c-axis. Other crystal forms can be seen in Ref 3.
Any plane through the trigonal lattice is represented by four numbers (hkmi). These are the Miller Indices which are the reciprocal values of the intercepts respectively on the a1, a2, a3, and c-axis. A family of planes is indicated by the notion {hkmi}.
Calcite forms twinned crystals according to four twin laws [Figure 4 in Ref 5] as shown in Figure 2. The twin forms and the associated family of planes are given in Table I. The angles between the vertical c-axes for each twin form are respectively to the nearest degree of 180, 127, 90, and 53. Calcite specimens exhibiting the twin laws are shown in Figures 5-8.
Among minerals calcite can be considered to be the best one to demonstrate cleavage because of its perfect cleavage along the rhombehedral family of planes {10-11}. The rhombehedral shape of the specimen demonstrating birefringence (double refraction) is evident in Figure 10.
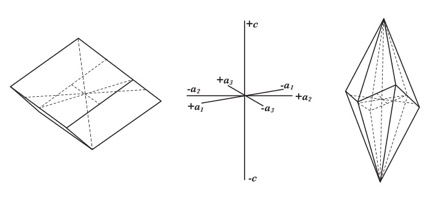
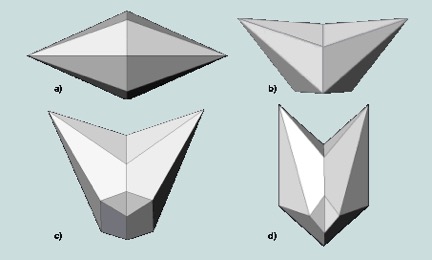
TABLE 1
| Twin Form | Family of Twin planes |
| a | {0001} |
| b | {10-11} |
| c | {01-12} |
| d | {02-21} |

Elmwood Mine, Carthage County, Tennessee [Ref 6].

(10-11) [Ref 7].


Brushy Creek mine, Reynolds County, Missouri [Ref 9].
Optical Properties
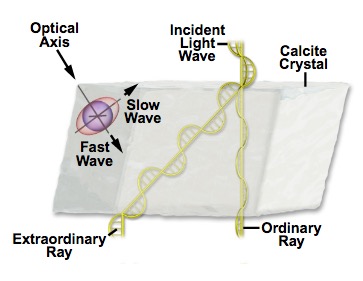
Calcite exhibits a range of lusters between vitreous to pearly and its transparency ranges between transparent to translucent. Calcite also exhibits birefringence in its refraction of light within the crystal [Ref 3]. Because the refractive index of calcite varies with the direction of light within a calcite crystal the light entering the crystal is doubly refracted into two different directions, as shown schematically in Figure 3 and demonstrated by the double image of the lines seen in Figure 4.

Sources of Color in Calcite
In its description of the properties of calcite, the mineral reference site mindat lists besides white, a rainbow of colors: yellow, red, orange, blue, green, brown, grey, etc. for calcite.
Calcite, which chemically is calcium carbonate with the formula CaCO3, is colorless when pure, as shown by the crystals on matrix shown in Figure 7 [Ref 12]. The rich colors of calcite specimens arise from different sources, such as substitution of low levels of one of the transition metals its divalent ionic form M+2for the calcium ion Ca+2in the lattice of the crystal, low densities of radiation-induced defects in the lattice of the crystal, or inclusions of a pigmented mineral.
Colors due to transition metal impurity ions [Ref A]
The colors present with iron and chromium, manganese, cobalt, or chromium present in, respectively ferroan, manganoan, cobaltoan, and chromian calcites are summarized in Table II. The yellow, pink and green colors due to these transmission metals can be seen also in many other minerals as can be seen in a search on the Web.
TABLE II
| MINERAL | COLOR |
| Ferroan Calcite | Yellow |
| Cobaltoan Calcite | Pink |
| Manganoan Calcite | Pink |
| Chromian Calcite | Green |
Colors arising from radiation damage in the crystal lattice
The search on the web for what colors of calcite might stem from radiation damage disclosed two references in which blue and amber-colored calcite were associated with radiation damage. Results of the study of References suggested that radiation-induced color centers involving the negative ion CO-3and the presence of stress-induced twInning and lattice disloctions account for the coloring mechanism.
The Mineral Spectroscopy Server of the Divisions of Geological and Planetary Sciences of the California Institute of Technology states that natural radiation induces an amber color in the Calcites from the lead-zinc Tri-State Mining District [Ref B, C]
Colors arising from colored inclusions
Colorful calcite is also produced by the presence of pigmented inclusions. Clear and transparent crystals of calcite, when included by such strongly colored minerals as malachite, pyrite, hematite, native calcite and others, make highly aesthetic specimens. The inclusions may be dispersed in the cystal (Figure 16) or reside on an included phantom crystal within the specimen crystal (Crystal 17).





Color due to an included pigmented mineral