Lattice Defects, Impurities and Color
As stated in references 17, 18 of my DIAMONDS II blog, impurity atoms and lattice vacancy defects are responsible for the coloration of diamonds [Ref 1]. A lattice vacancy (V) without a carbon atom in the diamond lattice which partners with one to four neighboring nitrogen atoms (N), in colored diamonds, as well as the space taken by an adjacent pair of them partnering with a nickel atom (Ni), are present in colored diamonds. These atom-vacancy structures are shown in Figures 1 to 6 of this Blog. Boron atoms (B), substituting for carbon atoms in the crystal lattice, give rise to the blue color. Hydrogen atoms, possibly associated with lattice vacancies, and nitrogen atoms may also be responsible for imparting color [Ref 2].
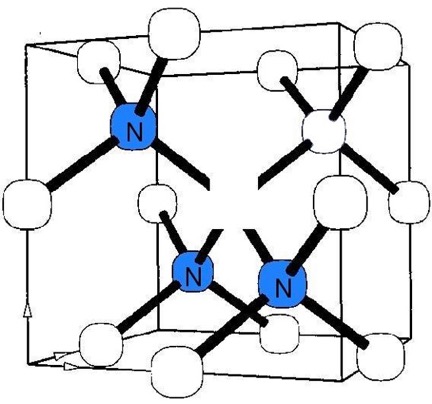
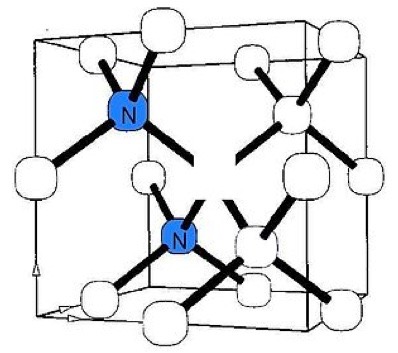
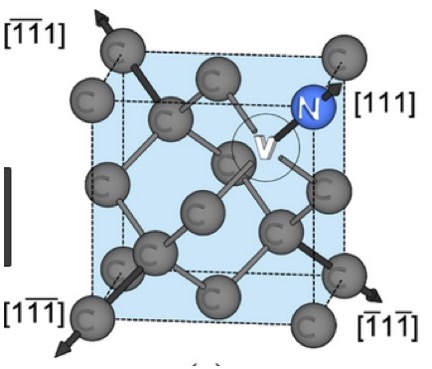
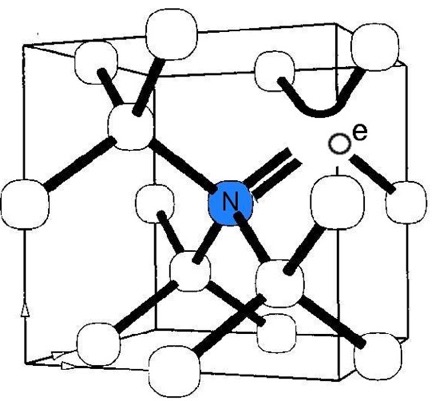
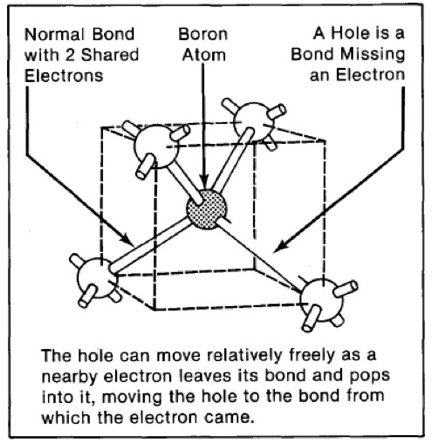
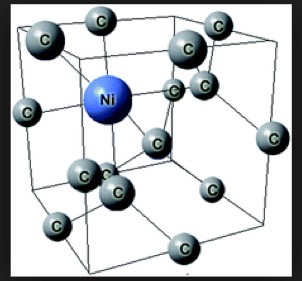
[Ref 6].
References for the N3-nitrogen center, the H3-nitrogen center, the NVO-nitrogen center, the NV-1-nitrogen center, the boron atom substation for a carbon atom, and the Nickel Di-vacancy center are described respectively in Refs 3, 4.
The colors of diamonds associated with these lattice defects and impurities are referred to in my DIAMONDS II Blog and shown below in Table I.
TABLE I
| DEFECT/IMPURITY | DIAMOND COLOR |
| N3-nitrogen center | Yellow |
| H3-nitrogen center | Green |
| NVO-nitrogen center | Pink |
| NV-1-nitrogen center | Pink |
| Boron atom substation for carbon | Blue |
| Nickel Di-vacancy | Green |
| Uncertain defect due to Hydrogen | Grey-brown, Yellow, Pink |