
In some minerals color is directly related to a metallic element, is characteristic, and can be useful in identification. As examples, azurite as shown in
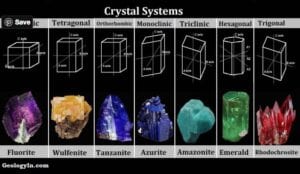
The atoms within the crystal of a mineral are arranged in a regular fashion to form a lattice, and the crystal exhibits a shape with

Cleavage in a mineral is the tendency for the crystal to split along definite crystallographic planes as exemplified by the rhombohedron cleaved from a calcite

Fracture in mineralogy is the texture and shape of the surface formed when the mineral is fractured. Fracture differs from cleavage and parting, which involve

o the great advantage of the beauty of their art, Chinese carvers of jade were guided by themes and decorative motifs in the shaping of

From the late Neolithic Age (circa 3500 BC-2070 BC) into today the crafting of jade art objects in China has produced beautiful and magnificent art
Since ancient times jade[Ref1] has been used by artisans to create beautiful jewelry and works of art. Art objects of jade have been carved in
In this Blog I’ll describe how biomaterials including animal and plant fossils are included and preserved in amber formed from tree resin, which by its
Amber is a hard resin formed from tree sap by fossilization and is many millions of years old[Ref1]. Since Neolithic times (about 9000-3000 BC) and
The mission of the Coconino Lapidary Club is to educate and inspire a desire to explore, collect, cut, polish and create with gems, minerals, and fossils.
©2023 All rights reserved. Coconino Lapidary Club
Custom crafted by NAZwebz Studio